
|
|
|
|
|
Reagenti per Ricerca su COVID-19:
|
|
|
|
|
 |
 |
 |
|
Anticorpi Ricombinanti |
Spike-ACE2 Inhibitors Screening Kit
ACE2 Blocking Antibody |
Librerie di antivirali, Kits |
|
|
|
|
 |
 |
 |
|
ACE2 Inhibitor Screening Assay Kit |
Coronavirus PCR ed molto altro |
Immunità Innata |
|
|
|
|
 |
 |
 |
|
ELISpot, FluoroSpot, ELISA |
Interferon ELISA |
Reagenti per Ricerca sui Vaccini |
Solo per Uso di Ricerca - Non si vende a Privati

Development & Characterization of Recombinant Human COVID-19 Antibodies

Ora disponibili a catalogo i seguenti Anticorpi Umani Ricombinanti:
AbFlex® Recombinant Antibodies
ELISA Pairs in Sandwich ELISA
For use in a plate-based sandwich ELISA, the following antibodies are recommended for capture (plate-coating) and detection. Note that antibodies recommended for detection are non-conjugated so that a secondary antibody which detects human IgG1 will be required.
Additional SARS-CoV-2 Products
In addition to the recombinant antibodies targeting the SARS-CoV-2 spike S1 protein, we also offer many purified recombinant proteins expressed by the COVID-19 virus. These recombinant SARS-CoV-2 proteins are in stock and ready to ship, and are available in up to 1 mg quantities. Contact us to inquire about bulk orders.


Includes False Positive Control Plate
This assay (AG-45B-0020-KI01) is an indirect ELISA assay based on Spike (RBD) coated protein, for qualitative measurement of human anti-SARS-CoV-2 IgG in serum and plasma. In this assay, a second plate (antigen non-coated Background Plate) is provided to measure the amount of IgG antibodies non-specifically bound to the well. To measure presence of anti-SARS-CoV-2 human IgG antibodies in serum or plasma, net sample optical densities (Net OD) are calculated by subtracting each sample Background Plate OD from the Spike (SARS-CoV-2) antigen plate (Spike Plate) OD.
This Sandwich ELISA Kit, detects human ACE2 (AG-45B-0023-KI01) in serum, plasma and cell culture supernatant. Sensitivity: 40pg/ml. Range: 0.0625 to 4ng/ml. Sample Type: Cell Culture Supernatant, Plasma, Serum.
The SARS-CoV-2 Neutralizing Antibodies Detection Kit (AG-48B-0002-KI01) contains key reagents required to test the presence of functional neutralizing antibodies against SARS-CoV-2 present in the serum or plasma. It is an easy and fast alternative to the classical neutralization assay using Vero E6 cells. This Detection Kit is based on a colorimetric reaction, which measures the binding of the RBD of the Spike S protein from SARS-CoV-2 to its human receptor ACE2. The presence of neutralizing / blocking antibodies in the samples are detected by reduction of signal indicating the inhibition of the Spike-ACE2 binding.
Easy Tool to Screen SARS-CoV-2 Blocking Compounds
AdipoGen Life Sciences' SARS-CoV-2 Inhibitor Screening Kit (AG-48B-0001) has been developed as a High Throughput Screening (HTS) detection assay for the determination of SARS-CoV-2 blocking compounds. This robust Kit contains all necessary components, including the plate, TMB and wash buffers.
Proteins: BULK Quantities AVAILABLE from STOCK (please inquire)
Anticorpi in evidenza:
Unique! Blocks ACE2 (human) and does not affect the enzymatic activity:
Detects ACE2 by Flow Cytometry
anti-ACE2 (human), pAb - AG-25A-0042
anti-SARS-CoV-2 Spike Protein S1 (RBD), mAb (rec.) (Covi-1) (preservative-free) - AG-27B-6005PF
anti-SARS-CoV-2 Spike Protein S1 (RBD), mAb (rec.) (Covi-2) (preservative-free) - AG-27B-6006PF
Antiviral Compounds - Potential Small Molecule Therapeutics Against COVID-19:
Potent SARS-CoV-2 Replication Inhibitor
Remdesivir (AG-CR1-3713) is an adenosine nucleotide analog with broad-spectrum antiviral activity. It is a prodrug that metabolizes into its active form GS-441524 (AG-CR1-3722). The compound interferes with the action of viral RNA polymerase and evades proofreading by viral exoribonuclease (ExoN), causing a decrease in viral RNA production (RNA synthesis arrest), either by terminating RNA chains or causing mutations. Remdesivir is a promising drug in SARS-CoV-2 clinical trials to stop the spread of COVID-19. LIT: Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency: C.J. Gordon, et al.; J. Biol. Chem. (Epub ahead of press) (2020) | Remdesivir potently inhibits SARS-CoV-2 in human lung cells and chimeric SARS-CoV expressing the SARS-CoV-2 RNA polymerase in mice: A.J. Pruijssers, et al.; BioRxiv Preprint (2020)
Potent Viral Entry/Protease TMPRSS2 Inhibitor
Nafamostat . mesylate (AG-CR1-3731) is a cell permeable broad spectrum potent and reversible synthetic serine protease inhibitor. It is an inhibitor of the enzyme transmembrane serine protease TMPRSS2 and blocks the entry of SARS-CoV-2 (COVID-19) into lung cells in vitro, with roughly 15-fold higher efficiency than Camostat . mesylate (AG-CR1-3716), with an EC50 in the low nanomolar range. LIT: Nafamostat mesylate blocks activation of SARS-CoV-2: New treatment option for COVID-19: M. Hoffmann, et al.; Antimicrob. Agents Chemother. (Epub ahead of print) (2020)
|
Amastatin . hydrochloride |
AG-CP3-7003 |
100938-10-1 |
Viral entry, ANPEP (Aminopeptidase N) |
Viral Replication |
|
Camostat mesylate |
AG-CR1-3716 |
59721-29-8 |
Viral entry, TMPRSS2 |
Viral Replication |
|
Chloroquine . diphosphate |
AG-CR1-3721 |
50-63-5 |
Lysosome function, Zinc ionophore, Immunomodulation |
Viral Replication |
|
Hydroxychloroquine . sulfate |
AG-CR1-3720 |
747-36-4 |
Lysosome function, Zinc ionophore, Immunomodulation |
Viral Replication |
|
Darunavir |
AG-CR1-3712 |
206361-99-1 |
Papain-like viral protease (PLVP) |
Viral Maturation/Replication |
|
Darunavir . ethanolate |
AG-CR1-3724 |
635728-49-3 |
Papain-like viral protease (PLVP) |
Viral Maturation/Replication |
|
Ebselen |
AG-CR1-0031 |
60940-34-3 |
Main Protease (Mpro)/3C-like Protease |
Viral Transcription/Replication |
|
Favipiravir |
AG-CR1-3717 |
259793-96-9 |
RNA-dependent RNA polymerases (RdRps) |
Viral Transcription/Replication |
|
Lopinavir |
AG-CR1-3715 |
192725-17-0 |
Coronavirus endopeptidase C30 (CEP_C30) |
Viral Maturation/Replication |
|
Mycophenolic acid |
AG-CN2-0419 |
24280-93-1 |
SARS-CoV-2 papain-like protease (PLpro) |
Viral Replication |
|
Niclosamide |
AG-CR1-3643 |
50-65-7 |
Endosome acidification |
Viral Replication |
|
Niclosamide . ethanolamine |
AG-CR1-3644 |
1420-04-8 |
Endosome acidification |
Viral Replication |
|
Nitazoxanide |
AG-CR1-3723 |
55981-09-4 |
Viral hemagglutinin, Viral IE2 |
Viral Maturation/Transcription/Replication |
|
Oseltamivir . phosphate |
AG-CR1-3714 |
204255-11-8 |
Viral neuraminidase, Release of viral particles |
Viral Replication |
|
Remdesivir |
AG-CR1-3713 |
1809249-37-3 |
RNA-dependent RNA polymerases (RdRps) |
Viral Transcription/Replication |
|
GS-441524 Remdesivir Metabolite |
AG-CR1-3722 |
1191237-69-0 |
RNA-dependent RNA polymerases (RdRps) |
Viral Transcription/Replication |
|
Ribavirin |
AG-CR1-3719 |
36791-04-5 |
RNA-dependent RNA polymerases (RdRps), RNA capping activity, Viral mutation rates, Immunomodulation |
Viral Transcription/Replication |
|
Ritonavir |
AG-CR1-3683 |
155213-67-5 |
Coronavirus endopeptidase C30 (CEP_C30) |
Viral Maturation/Replication |
|
Ruxolitinib . phosphate salt |
AG-CR1-3645 |
1092939-17-7 |
JAK1/JAK2, Immunomodulation |
Viral Replication |
|
Ruxolitinib (free base) |
AG-CR1-3624 |
941678-49-5 |
JAK1/JAK2, Immunomodulation |
Viral Replication |
|
Shikonin |
AG-CN2-0487 |
517-89-5 |
Main Protease (Mpro)/3C-like Protease |
Viral Transcription/Replication |
|
Tofacitinib |
AG-CR1-3625 |
477600-75-2 |
JAK1/JAK3, Immunomodulation |
Viral Replication |
|
Tofacitinib citrate |
CDX-T0461 |
540737-29-9 |
JAK1/JAK3, Immunomodulation |
Viral Replication |
|
Umifenovir . HCl [Arbidol] |
AG-CR1-3718 |
131707-23-8 |
Viral entry, Fusion into host cells |
Viral Replication |
 |
 |
 |
 |
 |
|
SARS Covid InVitro Research Scarica PDF
|
Innate Immunity
|
Immune Checkpoint Reagents
|
Functional Antibodies
Blocking/Neutralizing Antibodies
|
Inflammasome Research
|


SARS-CoV-2 Screening Library – 9003509
Diverse set of compounds. SARS-CoV-2 targets include:
Main protease (3CLpro) | Spike glycoprotein | ACE2 (human) | RdRp (Nsp12) | Endoribonuclease (Nsp15) | Guanine-N7 methyltransferase (Nsp14) | PLpro (Nsp3) | ADP-ribose phosphatase of Nsp3 | BRD2 (human)
Tools to Study SARS-CoV-2-Host Interactions:

Read the Article: Tools to Study SARS-CoV-2-Host Interactions
Existing Therapeutics for Viral Disease Research:

Read the Article: Using Existing Therapeutics Against SARS-CoV-2
SARS-CoV-2 related Research Assay Kits
Citrullinated Histone H3 (Clone 11D3) ELISA Kit - 501620
NETosis Assay Kit - 601010
NETosis Imaging Assay Kit - 601750
Using Existing Therapeutics Against SARS-CoV-2
Featured Article from 2020-03-03
Note: The products listed in this article are for biomedical research only. They are not for human or veterinary use.
Currently, there are no approved drugs to treat the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection that causes coronavirus disease 2019 (COVID-19). Existing FDA-approved drugs that have a known favorable safety profile are being examined for strategies to treat the disease and fast-track a treatment plan. The influenza drug favilavir (favipiravir; sold for research use only under the name T-705) has just been approved as an investigational therapy, and the Ebola virus drug remdesivir is currently undergoing clinical trial in coronavirus patients in China. The rational selection of drugs already on the market is being made based on their ability to inhibit any proteins essential for virus-receptor interaction and/or viral life cycle.
SARS-CoV-2, formerly known as the 2019 novel coronavirus (2019-nCoV), is a positive-sense, single-stranded RNA virus. This virus shares 79.5% sequence identity with SARS-CoV and uses the same angiotensin converting enzyme 2 (ACE2) receptor as SARS-CoV as a mechanism of cell entry. ACE2 is highly concentrated in airway epithelial cells. An envelope-anchored spike protein mediates coronavirus entry into host cells by binding to the host ACE2 receptor and then fusing viral and host membranes. Coronaviruses, including SARS-CoV, Middle East respiratory syndrome coronavirus (MERS-CoV), and infectious bronchitis virus (IBV), fuse at the plasma membrane or use receptor-mediated endocytosis and fuse with endosomes, depending on the cell or tissue type. This virus-receptor interaction facilitates both cross-species and human-to-human transmission of the virus, allowing the viral genome to be delivered to the host cell cytoplasm for replication.
Virus-Host Fusion Inhibitors
The broad-spectrum antiviral arbidol blocks viral fusion with target membranes, prohibiting viral entry into cells. Because it targets a common critical step for viral replication, it is effective against numerous viruses, including influenza A, B, and C as well as hepatitis B and C. A study has revealed that arbidol can effectively inhibit SARS-CoV-2 infection at a concentration of 10-30 µM in vitro. Besides its antiviral action, arbidol has been reported to produce an immunomodulatory response by inducing interferon production and stimulating the phagocytic function of macrophages.
Blocking virus-host fusion through inhibiting the Abl kinase pathway is also a promising target for the development of antiviral therapies, since this kinase activity is required for entry of coronaviruses. Abl kinases are composed of distinct domains that enable them to act as scaffolds for signaling complexes and to regulate protein function through phosphorylation of downstream targets. Pathogens have been shown to exploit Abl kinase signaling to rearrange F-actin cytoskeleton and trigger phosphorylation of viral effector proteins to facilitate viral-host fusion. A high-throughput screen identified imatinib as an inhibitor of SARS-CoV and MERS-CoV. It is likely that inhibition of Abl interferes with the actin dynamics required for virus-host fusion. Cayman offers several Abl kinase inhibitors that can be used to study the coronavirus membrane fusion step.
Abl Kinase Inhibitors
Imatinib (mesylate)
Saracatinib
GNF-2
GNF-5
See all Abl family & Bcr-Abl inhibitors
In addition to the coronavirus entering the host by binding to the host cell’s ACE2 receptor, participation of ACE in the renin-angiotensin system has been implicated in the acute, accelerated lung fibrosis associated with coronavirus infection. ACE mediates the conversion of angiotensin I to angiotensin II, which interacts with angiotensin II type 1 (AT1) receptors. In some pathological conditions, overactivation of AT1 receptors may lead to damaging events like fibrosis in the liver and lungs, possibly through increasing TGF-β expression. Presumably, a drug that would inhibit ACE, such as lisinopril, or block AT1, like losartan, would have a beneficial effect of mitigating the heavy fibrosis associated with acute cases of SARS infections by shutting down the ACE-angiotensin II-AT1 pathway. ACE inhibitors may further play a role in disallowing viral fusion of the coronavirus to the host cell and entry into the cell, denying its pathway to replication.
See all ACE inhibitors
See all AT1 receptor antagonists
Protease Inhibitors
After infection, genomic RNA is released into the cytoplasm of the host cell and translated into two long, overlapping polyproteins, pp1a and pp1ab, which are processed by two proteases, the main protease (Mpro or 3C-like protease) and the papain-like protease (PLpro). The hydrolytic activity of these proteases produces multiple functional proteins that are essential to forming the replicase complex for viral replication. Protease inhibitors block the viral life cycle by selectively preventing such proteolytic cleavage. The protein sequences of SARS-CoV Mpro and SARS-CoV-2 Mpro are 96% identical, indicating that protease inhibitors that have shown success against SARS-CoV should have similar efficacy against SARS-CoV-2. Both mycophenolic acid and the hepatitis C virus (HCV) protease inhibitors telaprevir, boceprevir, and grazoprevir have all been shown to bind to the active site of SARS-CoV-2 PLpro and hence, may be useful in preventing viral replication. A molecular docking study also revealed the HIV protease inhibitor indinavir to nearly perfectly overlap the region of the protein pocket of Mpro. Some success has already been shown in treating SARS-CoV-2-infected patients with the HIV protease inhibitors lopinavir and ritonavir in combination with the influenza neuraminidase inhibitor oseltamivir. However, a clinical study aimed to compare arbidol and lopinavir/ritonavir treatment for patients with COVID-19 with lopinavir/ritonavir only, supported lopinavir/ritonavir therapy but not lopinavir/ritonavir plus arbidol therapy. Cayman offers several protease inhibitors that can be used in candidate screens for inhibitors of Mpro and PLpro activity.
See all protease inhibitors
RNA-Dependent RNA Polymerase Inhibitors
Once inside the host cytoplasm, the single-stranded RNA virus serves as an RNA template that is replicated into complementary strands through the action of the RNA-dependent RNA polymerase (RdRp). The initiation step of RNA synthesis involves the addition of a nucleoside triphosphate to the 3’ end. The strand is elongated by repeated nucleotidyl transfer reactions with subsequent nucleoside triphosphates added to generate the complementary RNA. A class of nucleotide analogs have been developed as antiviral drugs to confuse RdRp as they are incorporated into RNA strands and induce non-obligate RNA chain termination. During the 2003 SARS outbreak, the RdRp inhibitor ribavirin in combination with the HIV protease inhibitors lopinavir and ritonavir was shown to reduce the disease course of clinical trial patients. BCX4430 (galidesivir) is in an advanced development stage under the FDA Animal Efficacy Rule to counteract viral threats from coronaviruses, flaviviruses, filoviruses, paramyxoviruses, togaviruses, bunyaviruses, and arenaviruses.
Development of some nucleoside-based therapeutics for SARS-CoV infections has been hampered by their removal via a proofreading 3’-5’ exoribonuclease (ExoN), but Remdesivir, an adenosine nucleoside analog that demonstrates broad-spectrum anti-RdRp activities has been shown to evade ExoN surveillance. Remdesivir was originally developed to treat Ebola virus, but also shows promising efficacy against SARS-CoV and MERS-CoV in pilot studies with an excellent safety profile in clinical trials so far. Remdesivir was used to treat the first US patient infected with SARS-CoV-2, who recovered. It is in phase 3 trials in Wuhan patients infected with SARS-CoV-2, overseen by the China-Japan Friendship Hospital in Beijing (NCT04252664 and NCT04257656). Remdesivir is a prodrug that metabolizes into its active form GS-441524. Cayman offers the following RdRp-targeted drugs that can be used to study viral replication.
RdRp Inhibitors
BCX4430 (Galidesivir)
GS-441524
Remdesivir
Ribavirin
T-705 (Favipiravir)
See all RdRp Inhibitors
Oxysterol-Binding Protein Inhibitors
During viral replication, oxysterol-binding protein (OSBP) plays a vital role in producing the membrane-bound viral replication organelles that form at the membrane contact sites between the ER and Golgi. The antifungal drug itraconazole and the natural compound OSW-1, which is being investigated as an anticancer drug, have been identified as functioning through targeting OSBP. While the binding modality of itraconazole is not known, OSW-1 has been shown to affect binding to one of the two established OSBP ligand binding sites. OSW-1 induces a prolonged reduction of cellular OSBP levels and has been shown to inhibit enterovirus replication. Coronaviruses may also be a suitable target for OSBP-targeted compounds.
Oxysterol-binding Protein Inhibitor
Itraconazole
OSW-1
Endosomal pH Regulators
Viruses entering host cells by endocytosis require an acidic pH in endosomal vesicles for virus-host fusion and to carry out the replication process. The antimalarial agent chloroquine (phosphate) is a weak base that shows broad-spectrum antiviral activities by increasing the endosomal pH required for viral activity. It can impair the replication of viruses by interfering with endosome-mediated viral entry as well as the late stages of replication of enveloped viruses whose glycosylation step requires a low pH for enzyme processing. Chloroquine (phosphate) can also suppress the release of TNFα and interleukin 6, which contribute to inflammatory complications of viral diseases. In multicenter clinical trials conducted in China, chloroquine (phosphate) has demonstrated potent efficacy in treating pneumonia associated with COVID-19.
Endosomal Acidification Inhibitors
Chloroquine (phosphate)
Hydroxychloroquine (sulfate)
Conclusion
Various potential targets for development of COVID-19 therapeutics exist along the stages from when a positive-sense, single-stranded RNA virus infects host cells and replicates. With little time available for drug testing and development, the repurposing of approved pharmaceutical drugs provides the most immediate solution for addressing the COVID-19 outbreak. Indeed, knowledge gained from the previous SARS outbreak has placed researchers in an advantageous position of better understanding solutions of how to address long-term treatment of this newly identified coronavirus. With hundreds of antiviral compounds in our catalog and custom synthesis services at the ready, Cayman scientists are poised to support the development of an effective therapeutic strategy against SARS-CoV-2 infection.
Simplify Screening and Hit-Seeking
-
Consists of 5 plates with >360 antiviral-associated compounds
-
Supplied in a 96-well MatrixTM tube rack format as 10 mM stock solutions in DMSO
-
Includes a variety of compounds with antiviral activity against HIV, hepatitis B virus, hepatitis C virus, influenza, herpes simplex virus, and coronaviruses, among others
We can add or remove specific compounds and provide bar-coded vials
|
|
|
|
|
 |
Cayman Currents
Innate Immune Signaling
cGAS / STING
|
 |
Immunology & Inflammation Cayman
|
|
|
|
|
|

 |
BPS Bioscience continues to be a leader in providing products and services to help meet the urgent need for new drugs and vaccines to treat and prevent COVID-19. BPS Bioscience has quickly developed an entire portfolio of recombinant proteins, unique assay kits, pseudovirions, antibodies, and lentiviruses to help researchers understand the pathogenesis of viral disease and to advance research and development of therapeutic drugs and vaccines. Visit our website frequently to discover our newest product and service offerings. |
|
|
|
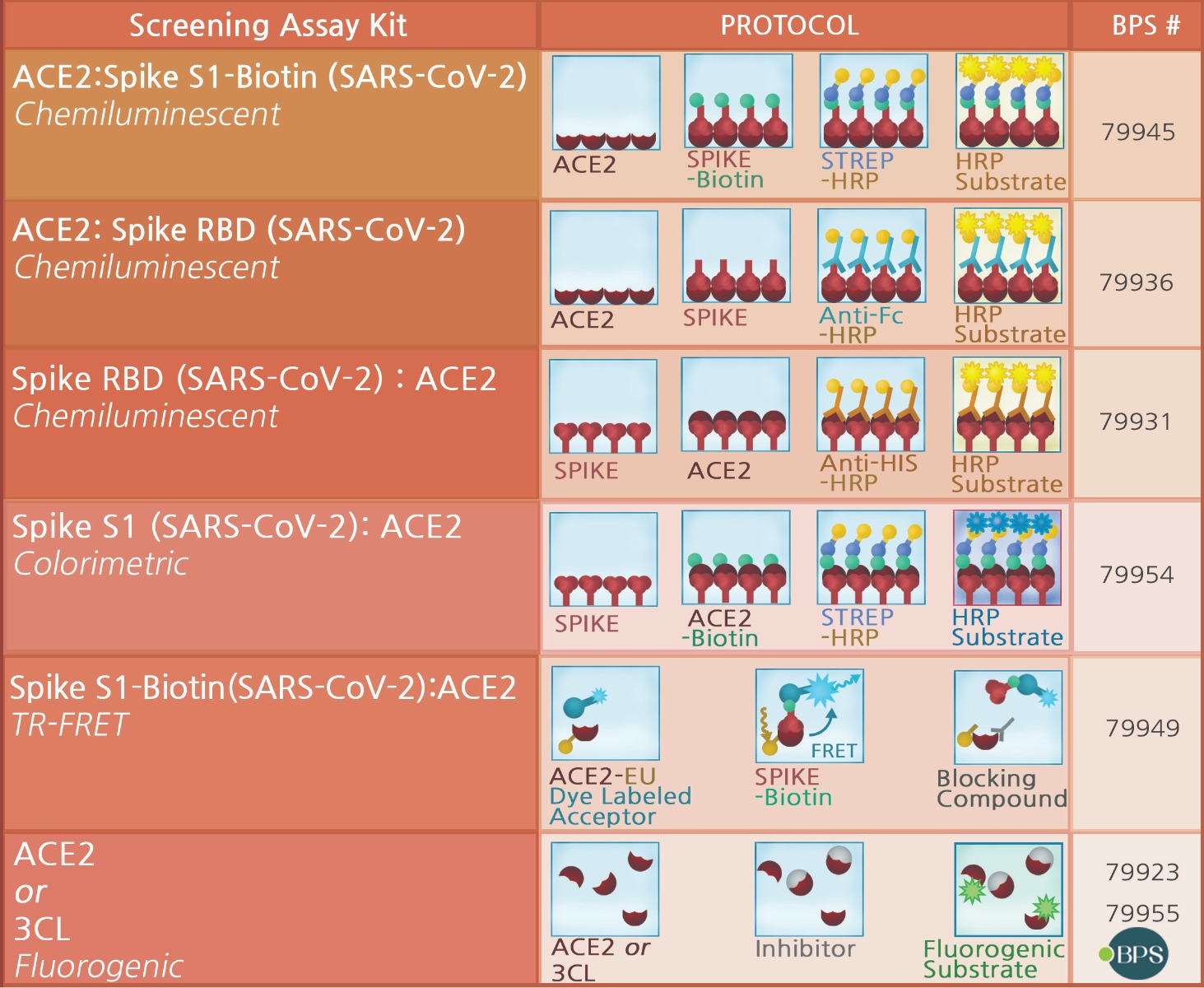
ACE2:SARS-CoV-2 Spike S1 Chemiluminescent Assay Kit - 79945
ACE2:SARS-CoV-2 Spike Inhibitor Screening Assay Kit - 79936
SARS-CoV-2 Spike:ACE2 Inhibitor Screening Assay Kit - 79931
Spike S1 (SARS-CoV-2): ACE2 Inhibitor Screening Colorimetric Assay Kit - 79954
3CL Protease Fluorescent Assay Kit - 79955
ACE2 Inhibitor Screening Assay Kit - 79923
Lentiviruses

|
Antibodies
|
Recombinant Proteins
|
|
|
|
|
|
|

ROLE OF COMPLEMENT IN COVID-19 INFECTED PATIENTS?
The innate immunity system could be important since it seems to be related in an inflammatory storm in COVID-19 infected patients, whereas complement appears to contribute to damage in lung. There are a few papers that show evidence and 2 small scale clinical trials are in preparation as found in public domain.
LITERATURE/CLINICAL TRIALS:
RELEVANT HYCULT BIOTECH PRODUCTS
 |
Hycult Elisa Kits
Scarica PDF
|
 |
Complement and collectins
ELISA kits, Antibodies and proteins for coagulation factors and complement proteins
C1q, C3a, C3c, MASP
|

ELISpot and FluoroSpot – methods for SARS-CoV-2 vaccine development
Mabtech supplies immune assays and instruments used to study immune responses, especially in vaccine development. The COVID-19 outbreak has increased the demand for several of our products, and many of our customers are currently doing research on or developing vaccines against SARS-CoV-2. We will continue to meet your urgent requests.
3850-4HPW-R1-1
ELISpotPLUS for enumeration of cells IgG antibodies specific for SARS-CoV-2 RBD
This kit is ideal for users who want a convenient and sensitive assay. The assay is designed for enumeration of B cells secreting IgG antibodies specific for the SARS-CoV-2 Receptor Binding Domain (RBD). Enumeration of all cells secreting IgG (total IgG) can also be made.
The kit includes one ELISpot strip plate pre-coated with monoclonal anti-human IgG antibodies, recombinant RBD-WASP (recombinant RBD with site specific peptide tag WASP), biotinylated detection mAb, anti-WASP-HRP, Streptavidin-HRP, polyclonal activators, and ready-to-use TMB substrate. Pre-coated plates reduce assay time and minimize variability.
For memory B cells, we recommend pre-stimulation with R848 and recombinant human IL-2 (both included).
3629-1
Peptide pool for stimulation cells specific for SARS-CoV-2 S1.
The SARS-CoV-2 S1 scanning pool is recommended for quantification of the number of cytokine secreting cells specific for SARS-CoV-2 S1. The peptide pool has been validated using human PBMC from COVID-19 convalescent individuals previously PCR-confirmed as SARS-CoV-2 positive.
Focus:
-
Human and mouse interferon gamma ELISpot enable monitoring of peptide specific T-cell responses in vaccine research, e.g. used in studies of SARS-CoV-1 (Li et al, J Immunol. 2008).
-
Cytokine ELISAs, ELISpot and FluoroSpot assays allow the measurement of cytokines, e.g. IL-6, GM-CSF and TNFalpha, involved in cytokine release syndrome associated with COVID-19 progression (Metha et al, Lancet 2020).
-
Monoclonal antibodies against immunoglobulins, and Ig ELISpot and FluoroSpot kits aid investigations of antigen specific B-cell responses (Jahnmatz et al, J Immunol Methods 2020).
-
Mabtech IRIS, our ELISpot/FluoroSpot reader facilitating unbiased spot counting, reliable multiplexing and measuring the relative spot volume.
Tuesday, March 31, 2020
 |
MabTech EliSpot
Find 1 cell in 100,000 with ELISpot
ELISpot is a sensitive assay used to quantify cytokine- or immunoglobulin-secreting cells at the single-cell level.
|
 |
Mabtech FluoroSpot
Discover more with FluoroSpot
FluoroSpot combines the sensitivity of ELISpot with the capacity to analyze secretion of several analytes simultaneously.
|
Ferret INF-gamma ELISpot Assay, ELISA Kit, Antibodies
Cat IFN-gamma ELISpot Assay, ELISA Kit, Antibodies
Soon available Chicken IFN-gamma ELISpot Assay and Antibodies

 |
PBL Interferon ELISAs
|
|
|
|
|
|
|
|
|
|
|
|
|